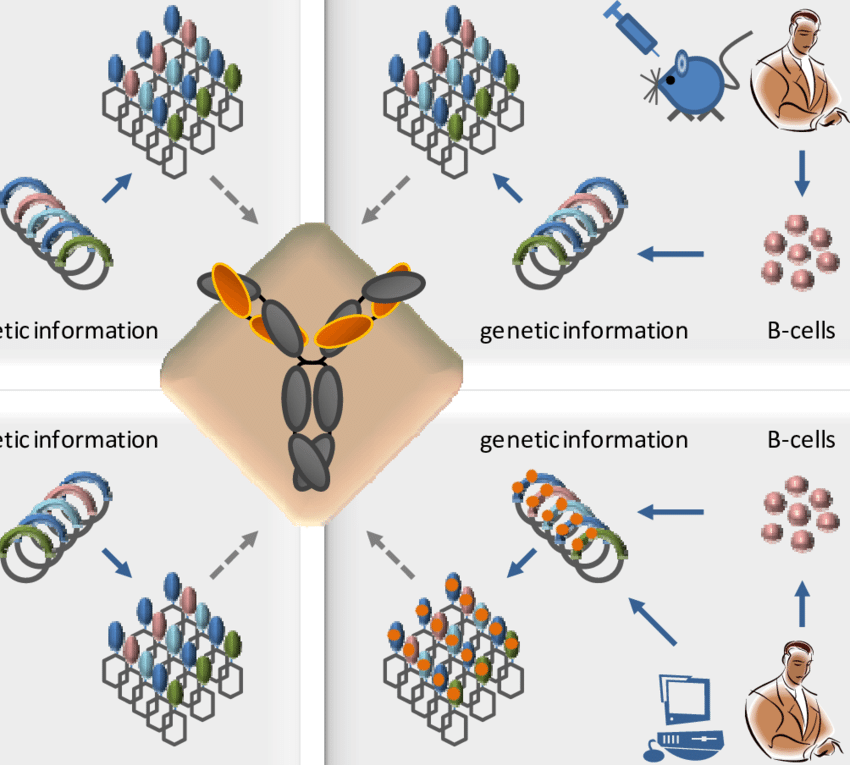
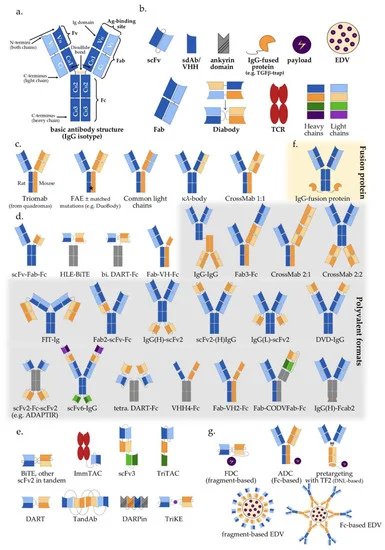
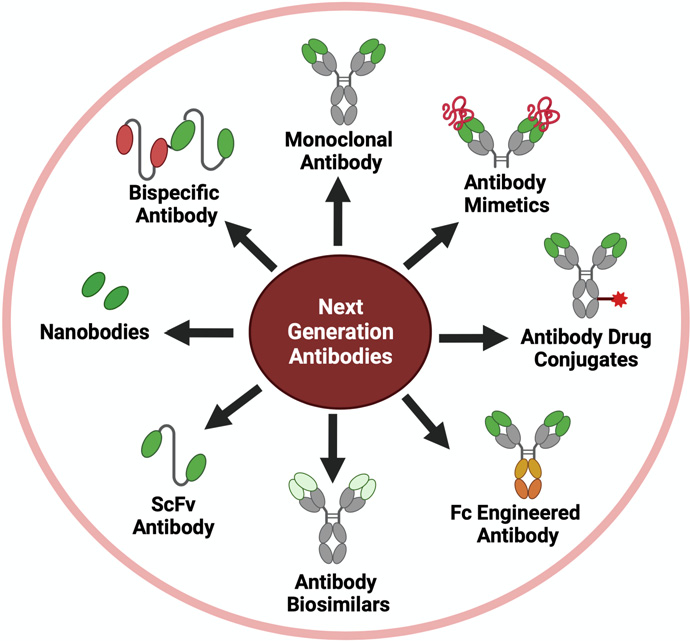
Introduction
Antibodies are fundamental tools in biomedical research, diagnostics, and therapeutics, but their natural evolution constrains their affinity, specificity, and stability. Next-generation antibody engineering leverages computational biology, directed evolution, and synthetic biology to enhance their properties beyond natural limitations. This blog explores emerging methods in antibody optimization, focusing on rational design, deep mutational scanning, machine learning-driven affinity maturation, and synthetic antibody libraries.
Rational Design of High-Affinity Antibodies
Structure-Guided Mutagenesis
Rational antibody design employs high-resolution structural data to guide sequence modifications that enhance binding affinity. Cryo-electron microscopy (cryo-EM), X-ray crystallography, and nuclear magnetic resonance (NMR) spectroscopy provide atomic-level resolution of antigen-antibody interactions. Key strategies include:
- Paratope optimization: Site-directed mutagenesis of complementarity-determining regions (CDRs) to introduce stabilizing interactions such as hydrogen bonds, electrostatic forces, and hydrophobic contacts.
- Framework stabilization: Engineering residues outside the CDRs to enhance structural rigidity and prevent conformational flexibility that reduces affinity.
- Glycan engineering: Modifying glycosylation sites to improve stability and half-life while preserving antigen binding.
Computational Affinity Maturation
Computational tools, including molecular dynamics (MD) simulations and free energy perturbation (FEP) methods, enable the in silico prediction of high-affinity variants. Key platforms include:
- Rosetta-based modeling: Predicts structural perturbations caused by mutations and optimizes paratope interactions.
- AlphaFold and antibody-specific AI models: Provides accurate three-dimensional structures of engineered variants.
- Docking algorithms (e.g., HADDOCK, ClusPro, AutoDock): Simulate antigen-antibody interactions to identify high-affinity candidates prior to experimental validation.
Deep Mutational Scanning and High-Throughput Screening
Comprehensive Sequence Space Exploration
Deep mutational scanning (DMS) enables the systematic analysis of large mutational landscapes by coupling high-throughput sequencing with functional selection. Techniques include:
- Yeast and phage display libraries: Introducing targeted mutations into antibody genes expressed on phage or yeast surfaces, followed by selection for enhanced affinity.
- Flow cytometry-based screening: Using fluorescence-activated cell sorting (FACS) to isolate cells expressing high-affinity binders.
- Next-generation sequencing (NGS) analysis: Mapping the impact of each mutation on binding affinity, stability, and expression levels.
Machine Learning-Assisted Affinity Maturation
Machine learning (ML) models trained on DMS data can predict beneficial mutations with higher accuracy than traditional random mutagenesis. Key approaches include:
- Supervised learning models: Predicting affinity changes based on labeled datasets of experimentally characterized mutants.
- Reinforcement learning algorithms: Iteratively optimizing antibody sequences based on experimentally validated feedback.
- Generative adversarial networks (GANs) and variational autoencoders (VAEs): Designing de novo antibody sequences with high affinity and stability.
Synthetic Antibody Libraries
Rationally Designed Synthetic Libraries
Synthetic libraries eliminate the constraints of immune-derived repertoires by incorporating engineered diversity into key CDR regions. Strategies include:
- Codon-optimized synthetic gene libraries: Encoding amino acid variants with enhanced stability and specificity.
- Non-canonical amino acid incorporation: Expanding the chemical diversity of antibodies using site-specific incorporation of synthetic residues.
- Scaffold engineering: Utilizing alternative frameworks, such as nanobodies and fibronectin-based scaffolds, to enhance stability and expression.
DNA-Encoded Antibody Libraries
DNA-encoded libraries (DELs) allow for the rapid synthesis and screening of billions of variants. This technology combines:
- DNA barcoding: Encoding each variant with a unique sequence tag to facilitate high-throughput selection.
- Affinity-based selection: Using immobilized antigens or cell-based screening to enrich high-affinity candidates.
- NGS-based deconvolution: Identifying enriched sequences through high-throughput sequencing.
Applications and Future Directions
Therapeutic Engineering
- Bispecific and multispecific antibodies: Engineered antibodies capable of engaging multiple targets simultaneously for cancer and infectious disease treatments.
- Immune checkpoint modulation: Designing antibodies that selectively modulate T-cell activation pathways.
- CAR-T cell optimization: Enhancing chimeric antigen receptor (CAR) binding domains for improved targeting efficiency.
Diagnostics and Research Tools
- Single-molecule binding analysis: Using high-affinity antibodies in nanopore sensing and optical tweezer-based force spectroscopy.
- Intracellular antibody delivery: Engineering nanobody-based intracellular probes for real-time cellular imaging.
- Point-of-care diagnostics: Designing ultra-sensitive antibodies for lateral flow assays and biosensors.
Next-generation antibody engineering integrates computational modeling, high-throughput mutagenesis, and synthetic biology to overcome natural limitations. Advances in rational design, deep mutational scanning, and machine learning are enabling the creation of high-affinity binders with unprecedented specificity and stability. These technologies are poised to revolutionize therapeutic antibody development, diagnostics, and fundamental research in structural biology and immunology.
Gentaur Antibody-related Products